Autoimmune connective tissue diseases1 (CTD) such as lupus, Sjögren’s syndrome, and systemic sclerosis present a diagnostic challenge due to overlapping symptoms and complex antibody profiles. At AliveDx, we’re committed to the challenge of simplifying that complexity through innovative in-vitro diagnostic solutions that support the improvement of rapid disease detection and clinical decision making for fast patient outcomes.
What are Anti-DFS70 autoantibodies?
Anti-DFS70 autoantibodies target a protein known as Dense Fine Speckled 70 (DFS70), also referred to as lens epithelium-derived growth factor (LEDGF). These antibodies are often detected during indirect immunofluorescence assays (IFA) on HEp-2 cells, where they produce a distinct speckled pattern. While the clinical relevance has been debated, emerging evidence suggests that a presence, especially in isolation, may indicate a lower likelihood of autoimmune CTD2.
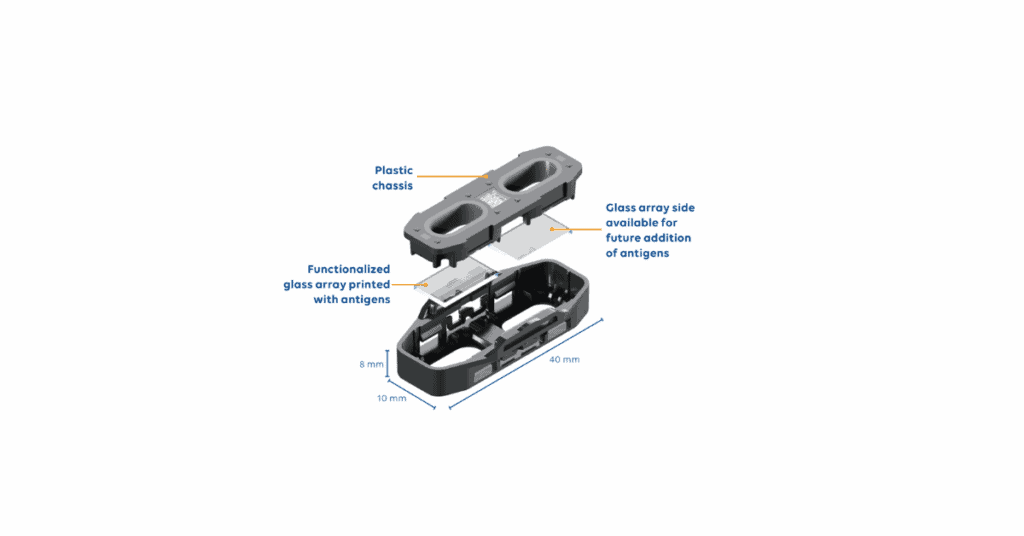
A new lens on autoimmune testing
In a recent study3 presented at the 17th Dresden Symposium on Autoantibodies (September 2025, Dresden, Germany), AliveDx researchers evaluated the clinical utility of anti-DFS70 detection using the MosaiQ AiPlex® CTDplus microarray assay. This fully automated, multiplexed immunoassay, simultaneously detects 15 IgG autoantibodies, including those associated with lupus (dsDNA, Sm, SS-A), scleroderma (Scl-70), and myositis (Jo-1), alongside DFS70.
Patient study highlights:
○ Sample Size: 667 patients with confirmed CTD, 558 disease controls, and 233 healthy blood donors.
○ Key Finding: Anti-DFS70 antibodies were found in:
But the real insight came from analyzing DFS70 in isolation versus DFS70 with other autoantibodies.
Empowering diagnostic insights:
○ Specificity: 100% for excluding CTD when DFS70 was present without other autoantibodies.
○ Sensitivity: 84.2% when DFS70 was present with other autoantibodies in CTD patients.
These results suggest that isolated DFS70 positivity may serve as a strong indicator against autoimmune CTD, helping clinicians avoid unnecessary follow-up testing and patient anxiety.

Why this matters for patients?
For patients undergoing autoimmune screening, a positive ANA (antinuclear antibody) test can naturally be of concern. However, not all ANA patterns point to a disease.
AliveDx’s MosaiQ AiPlex CTDplus assay provides:
A step towards a more personalized diagnostic
As autoimmune diagnostics evolve, the ability to interpret antibody profiles with nuance becomes increasingly important. AliveDx is proud to lead a shift towards in-vitro diagnostic automation, and solutions that supports the improvement in autoimmune disease testing and detection.
Whether you’re a clinician seeking diagnostic clarity or a patient navigating complex symptoms, our mission is to empower better decisions through smarter and more efficient patient testing.
Get in touch with us today to find out more about the power of in-vitro diagnostic solutions that are driven by innovation.
1Certain assays and intended uses for the MosaiQ® system are currently under development and are not yet cleared by the U.S. Food and Drug Administration. The MosaiQ® instrument is available in the U.S. as a class II 510(k) exempt device.
2 Data on file
3 Data on file
©2025 – AliveDx Suisse SA – AliveDx, AliveDx logo, MosaiQ, AiPlex and MosaiQ AiPlex are trademarks or registered trademarks of AliveDx group companies in various jurisdictions. This study was funded by AliveDx Suisse SA, Eysins, Switzerland. Not all methods may be available in all territories. Subject to regulatory clearance in some territories.





